Economy
Bars sold in Shaws, Stop & Shop, Price Rite, elsewhere recalled
According to the FDA, Fieldbrook Foods Corporation has issued a voluntary recall of all orange cream bars and chocolate coated vanilla ice cream bars that were produced in 2017

According to the FDA, Fieldbrook Foods Corporation has issued a voluntary recall of all orange cream bars and chocolate coated vanilla ice cream bars that were produced in 2017 on the company’s Hoyer 1 Line at its Dunkirk, NY plant (plant code 362677). This is the only production line and the only Fieldbrook Foods plant (of 3) involved in this recall notice. Both products are being recalled due to the possibility that they may be contaminated with Listeria monocytogenes, an organism which can cause serious and sometimes fatal infections in young children, frail or elderly people, and others with weakened immune systems. Although healthy individuals may suffer only short- term symptoms such as high fever, severe headache, stiffness, nausea, abdominal pain and diarrhea, Listeria infection can cause miscarriages and stillbirths among pregnant women.
The recalled orange cream bars, chocolate coated vanilla ice cream bars, and variety packs that include chocolate coated vanilla ice cream bars were sold at the following merchants under the indicated brands:
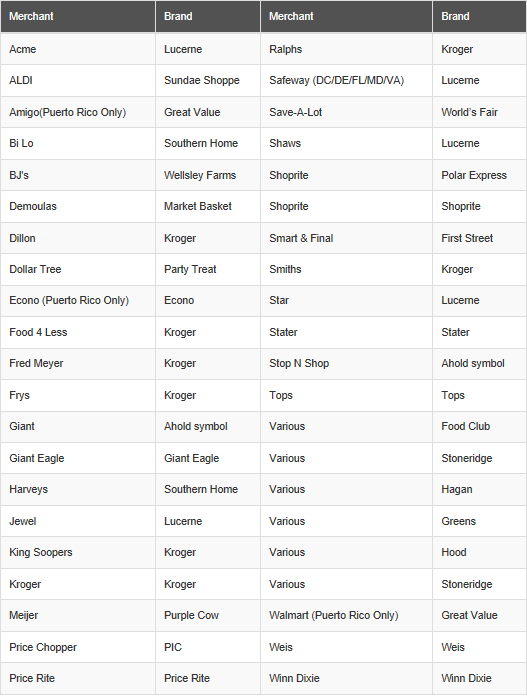
The recalled products have a production date of January 1, 2017 to December 31, 2017 and a “best by” date of January 1, 2018 to December 31, 2018. The Hood and Kemps products may show a “best by” date of July 1, 2018 to June 30, 2019. Fieldbrook Foods is working with each of these retailers to recall the affected products.
No illnesses have been reported to date in connection with this problem.
The potential for contamination was noted after routine industry testing revealed the presence of Listeria monocytogenes in only a few ice cream bar samples of many tested. The expansion of the recall is out of precaution for consumer health and food safety after a few additional samples tested positive for the presence of Listeria monocytogenes. There is no evidence of any contamination prior to October 31, 2017, but the company has issued the recall back to January 1, 2017 through an abundance of caution and in full cooperation with the FDA. The company has suspended production and distribution of all products produced on this production line while it cooperates with the FDA to fully investigate the source of the problem.
Consumers who have purchased these products are urged to return them to the place of purchase for a full refund. Consumers with questions may contact the company at 1 800/333-0805 x2270






You must be logged in to post a comment Login